How do mRNA vaccines work?
The vaccines work by teaching your immune cells to recognize a small piece of the SARS-CoV-2 virus called a spike protein. The SARS-CoV-2 virus is the virus that causes COVID-19.
Pfizer and Moderna use messenger RNA (mRNA)in their vaccines. The mRNA is a small piece of genetic code from the SARS-CoV-2 virus that tells the body to make the spike protein of the coronavirus. The production of the spike protein is recognized by immunity helpers, which will assemble an army of B cells. The B cells produce the antibodies that create immunity against the virus. After the vaccine causes this immune response, the body rapidly gets rid of the spike protein and the mRNA, the antibodies and immune memory remain.
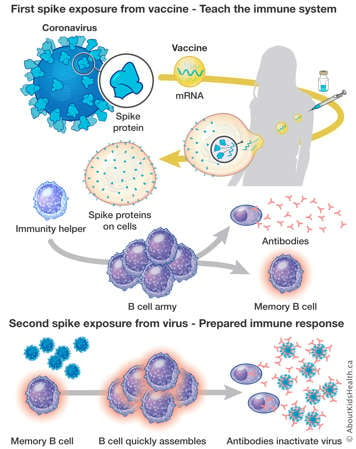
After vaccination, if your body encounters the coronavirus, the memory B cells recognize the spike protein on the virus and they will increase the antibody production. The antibodies will bind to the spike protein on the virus, blocking the virus from spreading.
Are mRNA COVID-19 vaccines safe and are there any side effects?
The mRNA vaccines available in Canada have met the requirements for approval by Health Canada, have been studied in clinical trials on a large number of people and were shown to be safe. In the studies, the number of people who got the vaccine and had unexpected severe side effects was similar to the number of people who received a placebo (substance or treatment that contains no active ingredients).
People who receive a COVID-19 vaccine may experience side effects, such as fatigue, headache, muscle pain, joint pain, chills and fever. These are side effects that are commonly seen after any vaccination. Allergic reactions have only rarely occurred after COVID-19 vaccination.
What about the risk of myocarditis and pericarditis?
Myocarditis (inflammation of the heart muscle) and/or pericarditis (inflammation of the sac that envelopes the heart) following a COVID-19 vaccine have been reported in Canada and internationally. These cases are very rare and are most frequently reported after the second dose of an mRNA vaccine. Most cases were mild and resolved with symptomatic treatment within a few days. As part of safety surveillance systems, Public Health Ontario is closely monitoring cases of myocarditis/pericarditis following COVID-19 vaccination.
Is there any chance that the COVID-19 vaccine can give me the virus?
No. There is no way you can get COVID-19 from any of the vaccines. None of the vaccines contain the SARS-CoV-2 virus, which causes COVID-19.
After vaccination, how long does it take to be protected from COVID-19?
After you get the vaccine, immunity usually starts to develop after 14 days. For vaccines that need additional doses, a maximum immune response occurs seven to 14 days after additional doses of the vaccine.
What is the difference between natural immunity and immunity from the COVID-19 vaccine?
Natural immunity refers to the immune responses that are developed following exposure to an infection. When contracting an infection, most individuals will develop antibodies that are key to recognizing and fighting the same infection, if encountered again. Natural immunity can decrease with time, and the antibodies may not last in your immune system for a very long time. Developing natural immunity also implies that you need to contract the infection, meaning that you could experience serious health complications as a result. For this reason, a series of COVID-19 vaccinations have been recommended since 2020 to help an individual’s immune system develop antibodies that protect against the virus.
Over time, past infection and the primary series of COVID-19 vaccination will not protect an individual from severe illness due to new strains of the virus. This is why COVID-19 vaccination is now recommended every fall.
Why is vaccination now recommended every year?
Although severe illness due to acute COVID-19 infection is less frequent in healthy children compared to adults, children can still be hospitalized and even require ICU-level care due to COVID-19. The same is true for influenza.
Vaccination helps prevent severe disease and hospitalization due to COVID-19. Annual COVID-19 vaccines protect against specific circulating strains of the virus that are increasing in frequency and causing illness. Updated annual COVD-19 vaccines are available in the fall season.
Another reason for vaccination is to get the virus under control during periods of high viral activity, which traditionally is during the respiratory virus season in the late fall and winter.
Can “long COVID” affect both children and youth?
Yes, it can. Cases of long COVID have been detected in both children and youth, although it appears less common than in adults. Early studies are showing a decreased risk of long COVID in adults who have been vaccinated, and this is promising for children and youth as well, however, more research is needed.
How many doses will my child need?
The timing and number of vaccine doses depends on your child’s age and vaccination status. Children who are known to have compromised immune systems and those who are receiving COVID-19 vaccines for the first time will follow a different schedule than those who are receiving one annual dose. This should be discussed with your child’s health-care team.
If your child is eligible to receive an updated vaccine dose and has recently had COVID-19, they can receive the dose at least 3 months and ideally 6 months after the infection.
Can I get other immunizations, such as the influenza (flu) vaccine, at the same time as the COVID-19 vaccine?
According to the National Advisory Committee on Immunizations, COVID-19 vaccines may be given at the same time as, or any time before or after, other vaccines, including the influenza (flu) vaccine.
If my child or I already had COVID-19, should we still get the vaccine? If so, when?
Yes. It is recommended that anyone who has had COVID-19 still get the vaccine. It is recommended to wait 6 months following infection for a vaccine dose, with a minimum interval of 3 months being acceptable. The clinical trials included people who previously had COVID-19, and the vaccine was found to be safe for them. Because antibodies against COVID-19 decrease after infection and it is possible to get the infection again (sometimes more severely), the vaccine is recommended as it can be helpful in boosting a person's existing immunity to COVID-19.
Will getting the COVID-19 vaccine help my child stay in school and in their extra-curricular activities?
All children and youth benefit from routine educational, physical and other extracurricular activities. School and other activities are likely to be able to stay open if hospitalization rates are manageable. Vaccination is the most important step to ensure this in adults and children six months of age and up.
My child is afraid of needles. What can I do to help?
Some children have a very strong reaction to needles. If your child is worried about getting a needle, you can ask for special ways to support their vaccination, such as a longer appointment time or a private space for the injection. The CARD system (Comfort, Ask, Relax, Distract) may also help. It provides groups of strategies to reduce the pain, stress and worries associated with vaccinations to make the experience a more positive one. More information can be found at AboutKidsHealth.ca/card. For children worried about pain, there are numbing creams and patches available at many pharmacies to help minimize needle discomfort.
What can I do for my child who is sensory-sensitive?
Ask your health-care provider to use the CARD strategies listed above to offer a calmer environment for your child, giving them as much time as they need and their own room to get the vaccine. Some clinics also offer sensory-sensitive appointments, offering dimmed lights, less noise and a slower pace, as well as privacy.
Can the COVID-19 vaccine affect puberty or fertility in children?
There is no evidence and no scientific reason to believe that the COVID-19 vaccine can affect puberty and fertility in children.
Is vaccination safe for children with food allergies?
Yes. There is no reason a child with a food allergy of any kind should not be vaccinated. Children with a history of allergy to foods, oral drugs, insect venom or environmental allergies can receive COVID-19 vaccines without any special precautions. If you are concerned about the possibility of an allergic reaction to any of the vaccine ingredients, please consult your child’s primary health-care provider.
Is there advice you would give to families with children who are immunocompromised or have disabilities and medical complexity?
Vaccination remains the best layer of protection against COVID-19 for everyone. It is important your child receives the vaccinations for which they are eligible. Children who are known to have compromised immune systems will follow a different schedule and this should be discussed with your child’s health-care team.
What is the current evidence for vaccination for COVID-19 in children with disabilities and medical complexity?
Current evidence suggests that children with disabilities and medical complexity may be at an increased risk for severe illness or complications from COVID-19 based on their underlying condition making vaccination and prevention of COVID-19 especially important.
What special considerations are there when vaccinating children with disabilities and medical complexity? Where can family caregivers find additional resources?
Families should consider different strategies that have worked well with previous immunizations and create a plan to set their child up for success. Some questions to consider when scheduling your child’s vaccination appointment include:
- Does my child require a calmer environment? (i.e., privacy, quiet)
- If applicable, is the vaccination clinic wheelchair accessible?
- Which distraction techniques are typically most effective for my child (i.e., deep breathing, counting, watching a favourite video, stress balls)
- What position will be most comfortable for my child during their vaccination (i.e., comfort holding, sitting with a caregiver, lying down)
If family caregivers have questions related to vaccinating children with disabilities and medical complexity, you should first reach out to your child’s primary health-care provider.
