What is 131I-MIBG therapy?
MIBG is short for metaiodobenzylguanidine, which is a substance that concentrates in neuroblastoma tumours. A type of radioactive iodine, called 131I, is combined with MIBG to form 131I-MIBG. The ¹³¹I-MIBG compound is designed to target neuroblastoma cells and deliver radiation directly to those cells in order to destroy them.
Why does my child need 131I-MIBG therapy?
Your child’s oncologist (cancer specialist) may recommend 131I-MIBG to treat high-risk neuroblastoma. This targeted radiation treatment is one way to destroy cancer cells and may be one part of your child's treatment plan.
Why does my child need to stay in the hospital?
131I-MIBG therapy is given through a central venous line or port-a-cath (these are types of intravenous (IV) lines), where it will enter the bloodstream and travel to neuroblastoma cells. Children who have received 131I-MIBG therapy will emit radiation. The radiation will leave the body mainly through urine but also through sweat, saliva and stool (poop).
The Canadian Nuclear Safety Commission (CNSC) requires patients receiving radiation above certain doses to stay in an isolated hospital room. This is to make sure that radiation safety regulations are strictly followed. SickKids adheres to these regulations to safely manage the radiation from this treatment, using special equipment and providing radiation safety training for all staff and family caregivers who care for patients receiving 131I-MIBG therapy. These added precautions help ensure that radiation exposure remains as low as reasonably achievable (ALARA) for the health-care team, caregivers and the public.
Risks and side effects of 131I-MIBG therapy
Your child may experience some or all of the following side effects of 131I-MIBG therapy:
- Nausea and vomiting
- High blood pressure (hypertension)
- Short-term loss of appetite
- Jaw pain and/or inflammation of the salivary gland and/or dry mouth
- Discomfort from the urinary Foley catheter
- Low blood counts—this will occur in the weeks following 131I-MIBG therapy and will be managed in outpatient care at your home centre
- Liver toxicity
- Late effects:
- Thyroid toxicity (hypothyroidism, hyperthyroidism or thyroid nodules)
- Growth failure
- Premature ovarian insufficiency (the ovaries stop working as they should) or problems with fertility
- Low risk of secondary malignancies (cancer)
Talk to your child's 131I-MIBG team if you have any questions about these side effects.
The health-care team will monitor and help manage any side effects of 131I-MIBG therapy, but let them know if you think your child is experiencing any of theses side effects while in the hospital or at home.
Preparing for 131I-MIBG therapy
Prior to and on the day of admission for 131I-MIBG therapy, you and your child will meet with the 131I-MIBG treatment team and the RSO to review the following:
- The benefits and risks of 131I-MIBG therapy
- What to expect during and after therapy
- Your roles and responsibilities
- Radiation safety precautions
A child life specialist will also meet with you and your child to plan activities for their admission. The child life specialist can also help prepare your child for the 131I-MIBG therapy through medical play or other developmentally appropriate activities.
Your child will need a variety of tests and procedures in the weeks before the 131I-MIBG therapy date (e.g., CT scan, MIBG scan, echocardiogram, kidney function test (GFR) and blood work). The night before the treatment, your child will start receiving a thyroid-protecting medicine to stop the thyroid from taking up 131I and to protect their thyroid gland from the radiation.
Your child may need a urinary catheter (also called a Foley catheter) to help drain their bladder because their urine will be radioactive. Your child will need a central venous line (CVL) or port-a-cath to receive the 131I-MIBG therapy. It may also be necessary to insert a peripheral intravenous (IV) line. Your child may also need a nasogastric tube to deliver medications (e.g., thyroid-protecting medicine) if they cannot take medications by mouth.
Some younger children may require sedation or other medications to keep them calm while they receive 131I-MIBG because family caregivers are not permitted in the MIBG suite during the infusion. For information on preparing your child for sedation, please see Sedation.
The 131I-MIBG suite
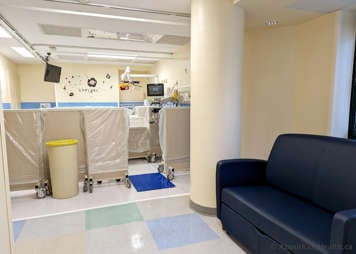
Treatment will take place in the hospital in a special room called the MIBG suite. The MIBG suite is a private room for the patient and caregiver with lead-lined walls and floor to minimize radiation exposure to others. The bed is surrounded by portable lead shields to further protect others from radiation exposure. The patient and caregiver area are also separated by lead shields.
Most high-touch surfaces will be covered in plastic to prevent surfaces from becoming contaminated with radiation. There are lead-lined containers for linen and “wet” garbage (diapers, emesis bags, etc.). Other waste bins are placed in the room to properly dispose of personal protective equipment (PPE) and medical or food waste. A lead lined box will also be placed near your child's bed. This will hold and shield your child's urine collection bag (Foley bag).
A radiation monitor is mounted to the ceiling of the room to continuously monitor how much radiation your child is emitting. There is a bathroom in the patient’s room with a toilet, sink and shower, which are only to be used by the patient. The RSO will let you know when your child is allowed to use the shower and the toilet.
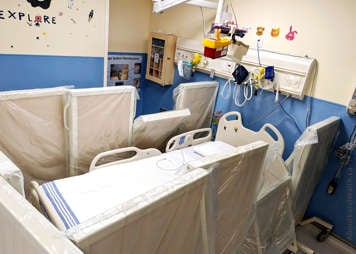

The 131I-MIBG infusion will be administered slowly over approximately 120 minutes. The nursing staff will prepare your child for the infusion, and a nuclear medicine technologist will administer the medication using an infusion pump. The technologist and a nurse will stay in the anteroom during the infusion to monitor your child. During the infusion, no one else is permitted to remain in the room.
While your child is receiving 131I-MIBG, they will need to stay in bed. They can play video games, watch movies and play with toys while the therapy is being infused.
What can my child and I bring with us?
You can bring your luggage and belongings into the caregiver area or anteroom, a room connected to but separate from the patient area. You will need to bring a pair of closed-toe shoes to be worn at all times. Do not bring personal items into the patient area without prior approval from the RSO. Items brought into the patient area may become contaminated with radiation after 131I-MIBG has been administered and may have to be stored at the hospital for up to three months. All personal items will be checked by the RSO before discharge and returned to you at a later date if found to have radiation contamination.
You should plan to leave anything at home that you do not want stored for prolonged periods of time. Any item your child handles—including clothing, blankets and toys—could become contaminated with 131I-MIBG. It is recommended that you have duplicates of special items that your child finds comforting so they will not miss them when they leave the hospital.
After treatment
Your child will be in radiation isolation as soon as the 131I-MIBG infusion begins. After treatment, most of the 131I-MIBG that is left in your child’s body will be eliminated through their urine over the next several days while your child is in the hospital. The side effects of 131I-MIBG could continue for several weeks after it has been given. Any side effects will be monitored by your home institution.
As you wait for the radiation to decrease to a level safe enough to go home, your child will need to stay in bed except when using the commode or toilet. Your child may play video games, watch TV or movies, play with toys, etc. Family caregivers will need to ensure that your child avoids touching things outside of the bed area. A family caregiver can stay with your child after the infusion is complete but will need to follow guidelines to minimize their radiation exposure.
Your child will be discharged from the hospital when radiation levels are at a safe level to follow precautions at home. The RSO will monitor radiation levels closely. Typically, the hospital stay is approximately five to seven days, but it could be longer. The health-care team and RSO will give you detailed discharge instructions for caring for your child at home and how to maintain radiation safety after discharge. These restrictions usually remain in place for a total of two weeks from the date of 131I-MIBG infusion. When you return home, your child will require regular follow-up care at your home institution. For more information about this, please see 131I-MIBG therapy: Caring for your child at home.
When to seek medical care (after discharge from the hospital)
131I-MIBG therapy can affect the bone marrow’s ability to produce normal blood cells. If this happens, your child may become prone to infections, bleeding and developing anemia.
Call your child's primary oncology team or go to the nearest Emergency Department right away if your child is experiencing any of the following:
- A fever of 38°C or more by mouth or 37.5°C or more under the arm
- Uncontrolled nausea or vomiting
- Prolonged bleeding or significant bruising
- Pale skin, tiredness and/or shortness of breath
- Difficulty breathing
- Appearing less alert
What to do if you have concerns
Contact your primary oncology team if you are concerned about your child. If your child presents to the hospital or Emergency Department within two weeks of receiving 131I-MIBG therapy, it is important to inform the health-care staff that your child has received the therapy and to contact the institution's radiation safety officer (RSO) for guidance.
References
Kayano, D., & Kinuya, S. (2018). Current consensus on I-131 MIBG therapy. Nuclear Medicine and Molecular Imaging, 52(4), 254–265. https://doi.org/10.1007/s13139-018-0523-z
Kumar, P., Cupit-Link, M., Federico, S.M., Bardwell, J.K., DuBois, S.G., Kao, P-C., London, W.B., Diller, L., & Henderson, T.O. (2025). Late effects in high-risk neuroblastoma survivors who received MIBG therapy. Journal of Clinical Oncology, 43, 10034. https.//doi.org/10.1200/JCO.2025.43.16_suppl.10034
Samim, A., Bleeker, G., Kraal, K. C. J. M., van Noesel, M. M., de Keizer, B., & Tytgat, G. A. M. (2024). A narrative review of 35 years of meta-[131I]iodobenzylguanidine therapy in neuroblastoma. EJC Paediatric Oncology, 3, 100159. https://doi.org/10.1016/j.ejcped.2024.100159